For Which Titration Is Phenolphthalein the Best Choice
Why universal indicator is not suitable for titration. What will be the pH of strong acid and weak base.
So option a is correct.
. In an acid-base titration neutralization occurs at pH 7. When you are performing a titration of a weak acid the best indicator choice to be used is phenolphthalein due to its pH range. What part of the curve corresponds to the optimum buffer action for the acetic acidacetate ion pair.
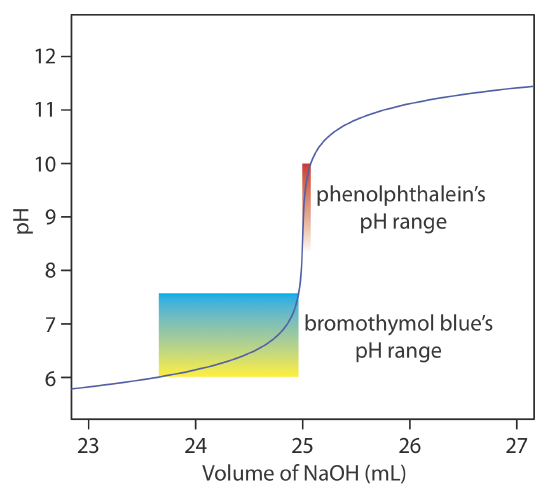
Bromocresel Green v Volame of ratien Phenclphthalein vs Volume of titration 2 EIS 795 95 105 Valume af titration 1 vell Volume of titratien 2 vo For which titration is phenolphthalein the best choice. If you look at the titration curve which plots the volume of base added vs pH source. The color of an indicator in a particular medium indicates whether it is acidic basic or neutral.
Phenolphthalein one of the most commonly used indicators shows a transition from colorless to magenta at a pH around 8. A strong acid- strong base titration is performed using a phenolphthalein indicator. For which titration is phenolphthalein the best choice.
For a titration of a weak acid against a strong base the titration curve is steepest a little above pH 7. For a titration of a strong acid with a strong base the titration curve is steepest at pH 7. You can see that the equivalence point occurs at pH 7.
Prepare 50 ethyl alcohol solution contained of 50mL ethanol and 50mL water. 2 N H C l is required. A the acidic solution of phenolphtalein is colourless while the basic solution is pink in colour.
Phenolphthalein is great for this titration. Explain your reasoning and support with evidence from your graphs Be specific. - For which titration is bromocresol green the best choice.
Then that would be. For applications in Chemical Pharma Food more we have Titrators to meet your needs. The indicator phenolphthalein whose range spans from pH 8 to 10 therefore makes a good choice for this type of titration.
In such titrations strong acid-weak base. When the number of moles of added base is equal to the number of. In the titration of strong acid and strong base phenolphthalein is used as suitable indicator.
Bromocresel Green v Volame of ratien Phenclphthalein vs Volume of titration 2 EIS 795 95 105 Valume af titration 1 vell Volume of titratien 2 vo For which titration is phenolphthalein the best choice. It will appear pink in basic solutions and clear in acidic solutions. Weigh out 05g of phenolphthalein.
A strong acid- strong base titration is performed using a phenolphthalein indicator. Phenolphthalein is fuchsia in pHs roughly between 82 and 12 and is colorless below pH 82. For a strong base-weak acid titration the equivalence point is probably near pH 9.
A strong acid- strong base titration is performed using a phenolphthalein indicator. What indicator is best for titration. Phenolphtalein is chosen because it changes color in a pH range between 83 10.
1 N H C l using phenolphthalein as an indicator. An indicator is an organic dye that can indicate the pH of a solution by color change. It is known as the titrant.
Methyl red Thus the best choice for weak base-strong acid titration is methyl red. It will appear pink in basic solutions and clear in acidic solutions. Ad Potentiometic Titrators for lab analysis with ready-made application methods.
What is an indicator. Phenolphthalein would be the best choice because its whole pH range is greater than 7 that is its pH range is 83 - 100 PAUSE Have you answered the question. A solution containing N a 2 C O 3 and N a O H requires 3 0 0 m L of 0.
Lets say that 2 drops of the indicator would be used for a titration. Methyl red is used as an indicator which changes the acidic solution to red in color which works in the range of pH from four to seven. For example if the pH at the equivalence point were 92 both thymol blue a phenolphthalein would be suitable indicators.
Phenolphtalein is chosen because it changes color in a pH range between 83 10. Methyl orange is then added to the above-titrated solution when a further 2 5 m L of 0. Reason In the titration of strong acid and strong base the equivalence point lies is the pH range of 30-105 and phenolphthalein have pH range of 80 - 98.
However it is still very steep either side of this and phenolphthalein will do the job. Now dissolve the phenolphthalein in the 50 ethyl alcohol solution. The equivalence point for the titration between strong acid and strong View the full answer.
If you dont know the pH change around the equivalence point of your titration consult a general chemistry textbook. For a strong acid-weak base titration the equivalence point is probably near pH 5. Since the pH versus concentration curve is so steep around the equivalence point any indicator that changes color in this general region can be used as an acid-base indicator.
PH Range of Color Change. So among the given options methyl orange and phenolphthalein can be used as indicators in acid base titration. Which is the best choice for weak base strong acid titration.
Here you could use an indicator like methyl red pH 44 to 62. The best indictor to use for a titration depends upon the pH at the equivalence point. If you dont know the pH change around the equivalence point of your titration consult a general chemistry textbook.
Thus the best choice for weak base-strong acid titration is methyl red. The pH range for phenolphthalein is 83 to 100. Show activity on this post.
If we consider titration curve for a acid -base titration it can be seen that the curve is very steep near the equivalence point. Again phenolphthalein is a good choice. The indicator range is 83-100 which is a good range while doing the titration of a weak acid with a strong base ie titratio View the full answer.
It will appear pink in basic solutions and clear in acidic solutions. This answer is not useful. - For which titration is bromocresol green the best choice.
Click to see full answer. Phenolphthalein The indicator phenolphthalein whose range spans from pH 8 to 10 therefore makes a good choice for this type of titration. Explain your reasoning and support with evidence from your graphs Be specific.
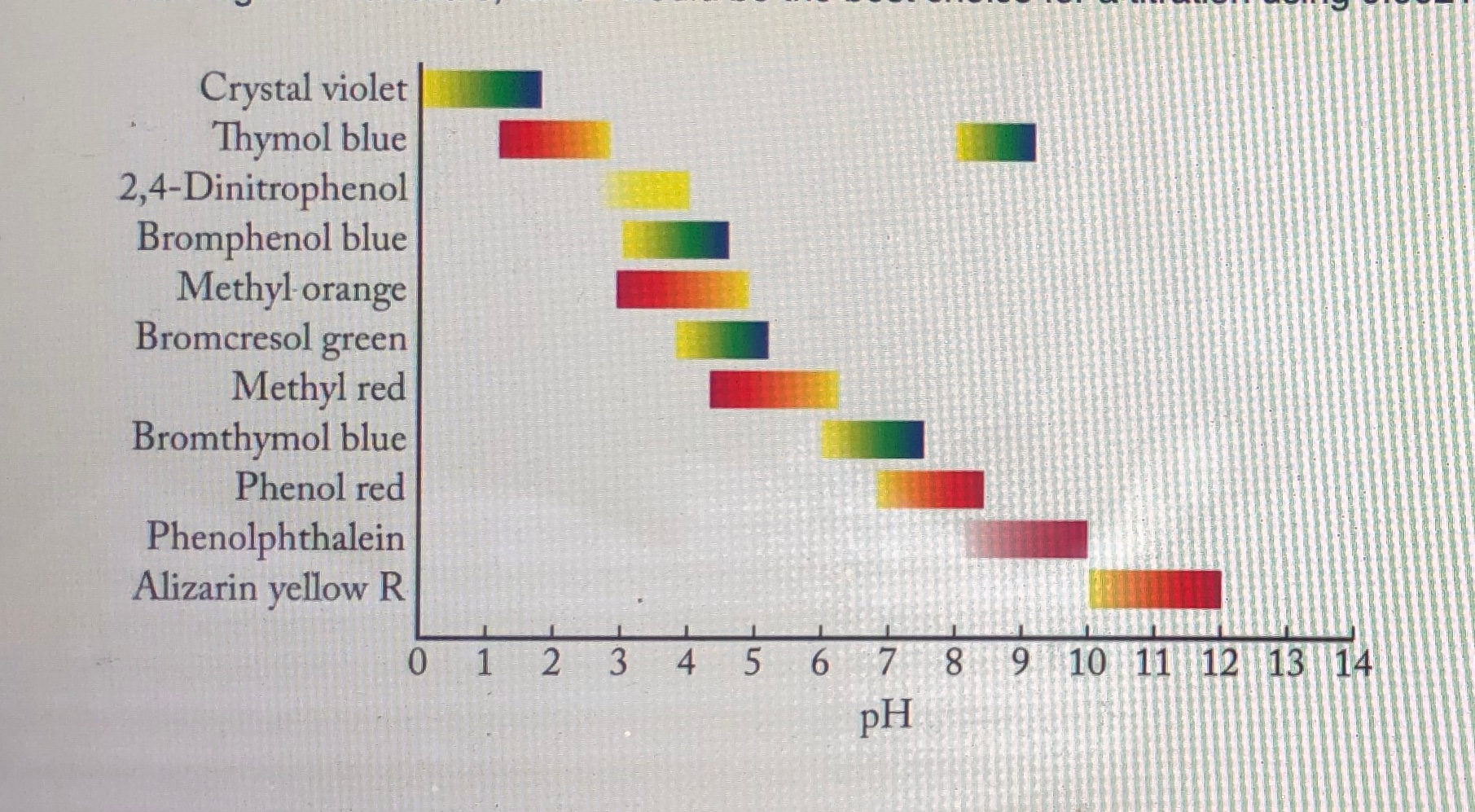
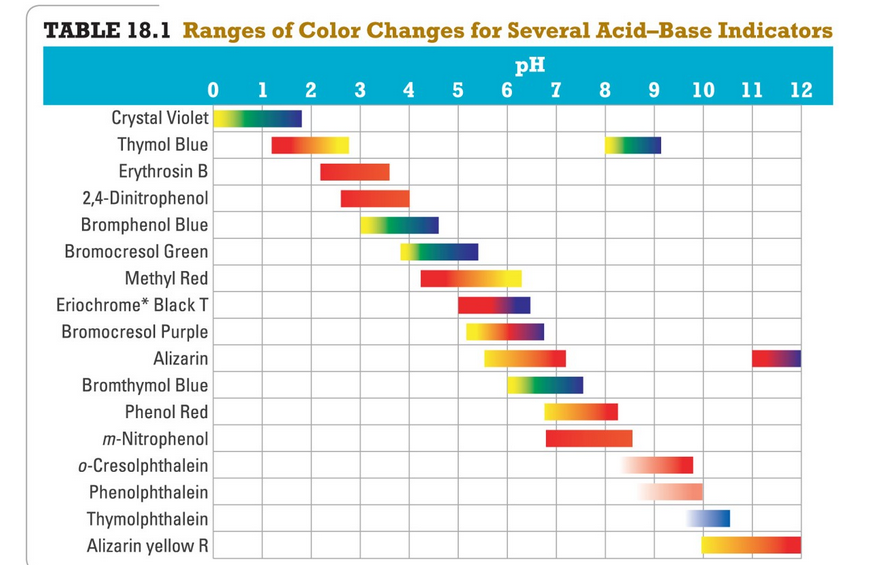
There is a variety of different color indicators available for determining the endpoint of a titration Table 1. Phenolphtalein is chosen because it changes color in a pH range between 83 10. Which of the following indicators is the best choice for this titration.
Store in dropper bottle for use.
9 2 Acid Base Titrations Chemistry Libretexts

Chemistry The Central Science Chapter 17 Section 3
Solved Based On The Chart Below Which Indicator Would Chegg Com
Why Is Phenolphthalein Not Used In The Titration Process Of Naoh And Hcl Quora
Why Is Phenolphthalein Used For The Titration Of Weak Acid And Strong Base Quora

Solved Q5 Which Of The Two Indicators Is A Better Choice Chegg Com

Question 1 6 Points Save Answer The Purpose Of An Indicator In An Acid Base Titration Is Homeworklib

Solved An Indicator Is Needed For A Titration If You Only Chegg Com
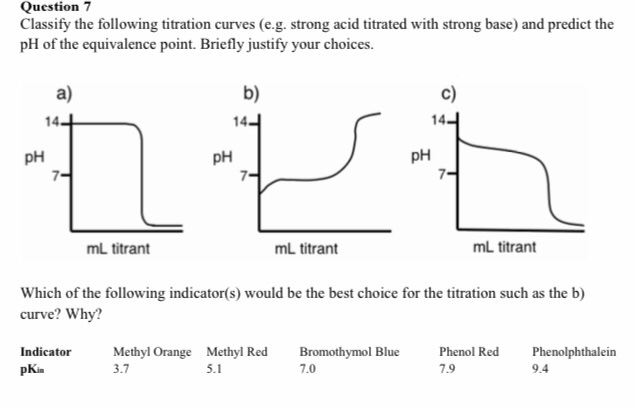
Solved Question 7 Classify The Following Titration Curves Chegg Com
Why Should The Colour Change When We Add A Base To An Acid In Titration Quora

Why Is The Phenolphthalein Used As An Indicator In The Titration Between Oxalic Acid And Sodium Hydroxide Quora

Acid Base Titration Titration Curves Equivalence Point Indicators Of Acid Base Titration

Solved 5 A 0 150 M Solution Of Aspirin Acetylsalicylic Chegg Com
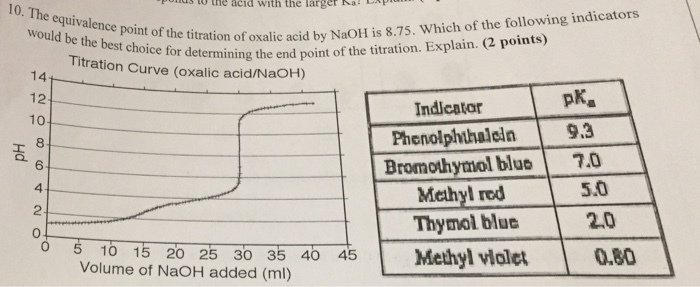
Solved 10 The S To The Acid With The Larger A Equivalence Chegg Com
Why Do We Use Phenolphthalein As An Indicator In Titration Quora

Which One Is The Best Indicator For Acid And Base Titration Between Methyl Orange And Phenolphthalein Quora

Acid Base Indicators Carolina Com
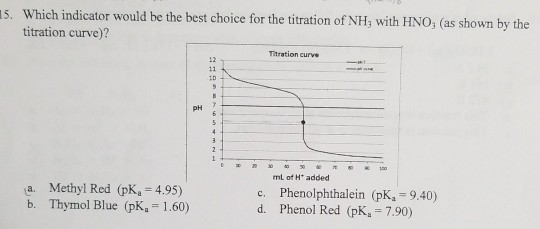
Solved Which Indicator Would Be The Best Choice For The Chegg Com


Comments
Post a Comment